Entropy
Practice Questions
AP Chemistry › Entropy
Page 1 of 2
10 of 12
1
Which of the following does not represent an increase in entropy?
2
Which of the following reactions represents a negative change in entropy?
3
A reaction will reach equilibrium when the system's entropy __________.
4
Which of the following scenarios describes a process with a negative entropy change?
5



What is the change in entropy for the following reaction?
6



What is the change in entropy for the following reaction?
7



What is the change in entropy for the following reaction?
8
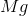

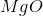
What is the change in entropy for the following reaction?
9




What is the change in entropy for the following equation?
10




What is the entropy for the following reaction?
Page 1 of 2

























