Enthalpy
Practice Questions
AP Chemistry › Enthalpy
H2O ice melts to liquid H2O \[Heat is added\]
Which of the following is correct?
I. Δ H is positive
II. ΔS is positive
III. ΔH is negative
IV. ΔS is negative
Calculate ΔH for the following reaction:
CH4 (g) + O2 (g) ⇌ CO2 (g) + H2O (l)
Compound ΔH
CH4 (g) -74.8 kJ/mol
H2O (l) -285.8 kJ/mol
CO2 (g) -393.5 kJ/mol
Which of the following is a true statement regarding the energy involved in the formation of Methane, CH4, from graphite C(s) and H2(g) ?
The formation of nitrogen dioxide is a two step process.


The net reaction is 
What is the change in enthalpy when creating four moles of nitrogen dioxide?
Which of the following statements is true concerning a chemical reaction?



What is the change in enthalpy for the given reaction?



What is the enthalpy of the following reaction?



What is the change in enthalpy for the following reaction?



What is the change in enthalpy for the following reaction?
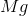

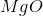
What is the change in enthalpy for the following reaction?





















