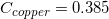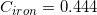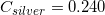Calorimetry, Specific Heat, and Calculations
Practice Questions
AP Chemistry › Calorimetry, Specific Heat, and Calculations
How much heat does it take to heat 100g ice at 0C to boiling point?
Cice= 2.1 J/goC
Cwater= 4.2 J/goC
ΔHvap= 2260 J/g
ΔHfus=334 J/g
A 50g sample of a metal was heated to 




Which of the following can be concluded?
Which of the following is the correct molar specific heat of water used when making calculations involving a calorimeter?
The specific heat capacity of an unknown liquid is 


In which instance would a bomb calorimeter be more useful than a coffee-cup calorimeter?
The following is a list of specific heat capacities for a few metals.
A 50g sample of an unknown metal is heated with 800 joules. If the temperature of the metal increases by 41.6oC, what is the identity of the unknown metal?
A 20g sample of iron at a temperature of 

What is the final temperature of the water?
How much energy is needed to raise the temperature of five grams of ice from 

You want to prepare a cup of tea. To do so, you pour 





How much heat is needed to raise